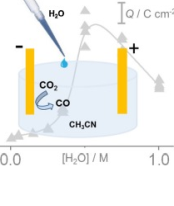
The promoting effect of water on the electroreduction of CO2 in acetonitrile
The paper authored by
A.V. Rudnev, U. Zhumaev, A. Kuzume,
S. Vesztergom,
J. Furrer, P. Broekmann and Th. Wandlowski
is published in Electrochimica Acta (2016, vol. 189, pp. 38–44).
Abstract:
The promoting effect of water on the electrochemical reduction of carbon dioxide (CO2) from non-aqueous solvents has been studied by means of cyclic voltammetry and in-situ surface-enhanced infrared absorption spectroscopy (SEIRAS). CO2 electroreduction on gold is known to be highly selective towards CO formation in aqueous and in non-aqueous media. The use of non-aqueous solvents is advantageous due to the significantly increased solubility of CO2 compared to aqueous systems. However, in the absence of any proton source, extremely high overpotentials are required for the CO2 electroreduction. In this work, we demonstrate for the first time a tremendous accelerating effect of water additives on the electroreduction of CO2 taking place at gold/acetonitrile interfaces. Already moderate amounts of water, in the concentration range of 0.5 to 0.7 M, are sufficient to decrease significantly the overpotential of CO2 reduction while keeping the CO2 concentration as high as in the pure acetonitrile. The effect of water additives on the mechanism of CO2 electroreduction on gold is discussed on the basis of electrochemical and IR spectroscopic data. The results obtained from gold are compared to analogue experiments carried out on platinum.
